
Author:XLIFESC
Release date:2023-08-09
Recently, the internationally renowned journal Cell Reports Medicine (IF=16.988) published the Phase I clinical research results of China's first TCR-T cell immunotherapy product, TAEST16001. The journal invited a professor from Northwestern University in the United States to provide a special commentary on the article, giving high praise and noting that the Phase I clinical research of TAEST16001 marks a critical step forward in the clinical translation of adoptive T-cell immunotherapy for advanced soft tissue sarcoma.


TCR-T cell immunotherapy involves obtaining peripheral blood mononuclear cell (PBMC) samples from patients, using genetic engineering to introduce high-affinity T-cell receptors (TCRs) into T cells, enabling them to recognize and attack tumor cells. These high-affinity TCR-T cells, once reintroduced into the patient's body, can seek out and specifically recognize tumor cells, releasing cytokines, perforins, and other agents to continuously kill tumor cells, thereby exerting anti-tumor effects.
TAEST16001 is a TCR-T cell immunotherapy product independently developed by XlifeSc. The first Phase I clinical trial was a dose-escalation and expansion study of TAEST16001 cells in patients with advanced soft tissue sarcoma who are HLA-A*02:01 positive and express the NY-ESO-1 antigen. The study was led by Professor Zhang Xing from the Sun Yat-sen University Cancer Center and Professor Fan Zhengfu from Beijing Cancer Hospital. The co-first authors of the article are Dr. Qiuzhong and Dr. Weng Desheng from the Sun Yat-sen University Cancer Center, and Professor Liu Jiayong from Beijing Cancer Hospital.
Professor Zhang Xing, as the co-corresponding author, stated that TCR-T therapy for soft tissue sarcoma is a clinically significant exploration direction. The Phase I clinical research results indicate that TAEST16001 cell therapy is well tolerated and shows anti-tumor activity in advanced soft tissue sarcoma expressing the NY-ESO-1 antigen. Researchers are looking forward to the ongoing Phase II clinical research of TAEST16001.
Article Highlights
• TAEST16001 cells are high-affinity NY-ESO-1 specific TCR-T cells.
• TAEST16001 cell therapy is well tolerated in advanced soft tissue sarcoma.
• TAEST16001 cell therapy shows promising anti-tumor signals.
• Multiple pre-specified biomarkers are associated with patient responses.
Article Abstract
NY-ESO-1 antigen-specific TCR-T cells are effective against tumors expressing the NY-ESO-1 antigen, but improvements are still needed in terms of safety and efficacy. Here, we report a first-in-human Phase I clinical trial using TAEST16001, an affinity-enhanced TCR-T cell therapy targeting NY-ESO-1. Patients received TAEST16001 cells after lymphodepletion with low-dose cyclophosphamide (15mg/kg/day for 3 days) and fludarabine (20mg/m²/day for 3 days), followed by low-dose interleukin-2 to maintain TCR-T cells in vivo. Among the 12 patients treated with this regimen, no treatment-related serious adverse events occurred. The objective response rate (ORR) was 41.7%, with a median progression-free survival (PFS) of 7.2 months and a median duration of response (DOR) of 13.1 months. The TAEST16001 regimen provides a safe and effective treatment for patients with advanced soft tissue sarcoma (ClinicalTrials.gov: NCT04318964).
Key Clinical Data
From March 23, 2020, to December 31, 2021, a total of 12 patients with advanced soft tissue sarcoma were enrolled (10 synovial sarcomas, 2 liposarcomas).
• Mean age: 33 years
• 83.3% of patients had received at least two prior lines of chemotherapy.
Safety Data
• TAEST16001 cell therapy was well tolerated, with no observed dose-limiting toxicities (DLTs) and no maximum tolerated dose (MTD) reached.
• The most common grade 3 or higher adverse events were lymphopenia (100%), neutropenia (92%), leukopenia (83%), and anemia (33%), mainly attributed to the lymphodepletion regimen. Other grade 3/4 toxicities included fever (8%), thrombocytopenia (8%), hypokalemia (8%), elevated alanine aminotransferase (8%), proteinuria (8%), and hypertriglyceridemia (8%).
• Two patients experienced grade 2 cytokine release syndrome, which resolved after symptomatic treatment. No neurotoxicity or serious infusion-related adverse events were observed.
Efficacy Data
• All 12 patients who received the specified TAEST16001 cell dose were evaluable for efficacy.
• At initial analysis, 5 of the 12 patients had a partial response, resulting in an ORR of 41.7% [95% CI, 15.2-72.3]; 5 patients (41.7% [95% CI, 15.2-72.3]) had stable disease, leading to a disease control rate of 83.3% [95% CI, 51.6-97.9] (Figure A).
• The median PFS was 7.2 months [95% CI, 2.5-11.8].
• As of the data cut-off date (April 15, 2022) (Figure B), 10 patients had experienced disease progression, and one patient had died due to disease. The median overall survival (OS) was not yet mature.
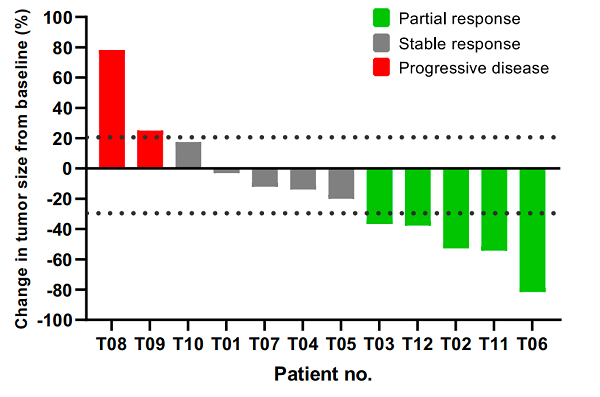
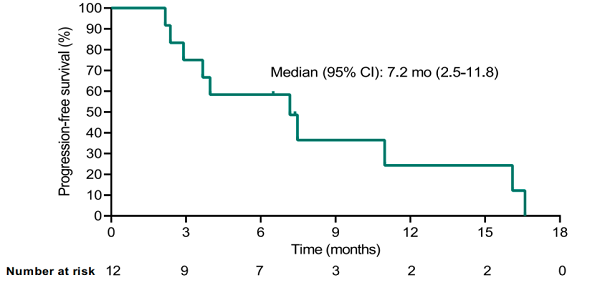
About TAEST16001
TAEST16001 is a TCR-T cell immunotherapy product independently developed by XlifeSc. It is the first TCR-T cell immunotherapy product in China to receive IND approval. At the American Society of Clinical Oncology (ASCO) 2022 annual meeting, Professor Zhang Xing was invited to present the Phase I clinical research data of TAEST16001 in the Sarcoma session. The results showed an objective response rate (ORR) of 41.7%, with safety and efficacy comparable to similar products from renowned international pharmaceutical companies, gaining recognition from peers and significant global attention.
About XlifeSc Ltd.
XlifeSc. (referred to as "Xiangxue Pharmaceutical") is a leading company in the field of TCR-T cell immunotherapy product and technology development. The company aims to "solve human health challenges and set a benchmark in cancer treatment," with the mission to "focus on TCR, empower T cells, and conquer solid tumors."
XlifeSc has independent intellectual property rights for its core TCR technology and a comprehensive TCR-T product R&D and manufacturing system. The TCR-T product R&D system includes: ① antigen peptide discovery platform, ② high-affinity TCR platform, and ③ TCR-T development platform; the TCR-T manufacturing system includes: ① automated cell production platform, and ② quality control platform. XlifeSc has established a complete innovation industry chain for TCR-T cell products. The company's R&D pipeline includes proprietary targets related to solid tumors and products covering the HLA types prevalent in the Chinese population, achieving a leading international level.
Currently, XlifeSc has two assets that have received IND approval in China. The first asset, TAEST16001, is indicated for soft tissue sarcoma and is currently in Phase II clinical trials. The second asset, TAEST1901, is indicated for primary liver cancer and is about to start Phase I clinical trials.
Publication Link:https://doi.org/10.1016/j.xcrm.2023.101133